Zircon
| Zircon | |
|---|---|
 A lustrous crystal of zircon perched on a tan matrix of calcite from the Gilgit District of Pakistan | |
| General | |
| Category | Nesosilicates |
Formula .mw-parser-output .nobold{font-weight:normal} (repeating unit) | zirconium silicate (ZrSiO4) |
| Strunz classification | 9.AD.30 |
| Crystal system | Tetragonal |
| Crystal class | Ditetragonal dipyramidal (4/mmm) H-M symbol: (4/m 2/m 2/m) |
| Space group | I41/amd |
| Unit cell | a = 6.607(1), c = 5.982(1) [Å]; Z = 4 |
| Identification | |
| Color | Reddish brown, yellow, green, blue, gray, colorless; in thin section, colorless to pale brown |
| Crystal habit | tabular to prismatic crystals, irregular grains, massive |
| Twinning | On {101}. Crystals shocked by meteorite impact show polysynthetic twins on {112} |
| Cleavage | {110} and {111} |
| Fracture | Conchoidal to uneven |
| Tenacity | Brittle |
Mohs scale hardness | 7.5 |
| Luster | Vitreous to adamantine; greasy when metamict. |
| Streak | White |
| Diaphaneity | Transparent to opaque |
| Specific gravity | 4.6–4.7 |
| Optical properties | Uniaxial (+) |
| Refractive index | nω = 1.925–1.961 nε = 1.980–2.015, 1.75 when metamict |
| Birefringence | δ = 0.047–0.055 |
| Pleochroism | Weak |
| Fusibility | close to 2,550 °C depend on Hf,Th,U,H,etc... concentrations. |
| Solubility | Insoluble |
| Other characteristics | Fluorescent and radioactive, May form pleochroic halos, Relief: high |
| References | [1][2][3][4][5] |
Zircon ( /ˈzɜːrkɒn/[6][7] or /ˈzɜːrkən/[8]) is a mineral belonging to the group of nesosilicates. Its chemical name is zirconium silicate, and its corresponding chemical formula is ZrSiO4. A common empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon forms in silicate melts with large proportions of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue and green. Colorless specimens that show gem quality are a popular substitute for diamond and are also known as "Matura diamond".
The name derives from the Persian zargun, meaning "gold-hued".[9] This word is corrupted into "jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from Zirkon, which is the German adaptation of this word.[10] Yellow, orange and red zircon is also known as "hyacinth",[11] from the flower hyacinthus, whose name is of Ancient Greek origin.
Contents
1 Properties
2 Applications
3 Occurrence
4 Radiometric dating
5 Gallery
6 Similar minerals
7 See also
8 References
9 Further reading
10 External links
Properties

Optical microscope photograph; the length of the crystal is about 250 µm
Zircon is ubiquitous in the crust of Earth. It occurs as a common accessory mineral in igneous rocks (as primary crystallization products), in metamorphic rocks and as detrital grains in sedimentary rocks.[1] Large zircon crystals are rare. Their average size in granite rocks is about 0.1–0.3 mm, but they can also grow to sizes of several centimeters, especially in mafic pegmatites and carbonatites.[1] Zircon is also very resistant to heat and corrosion.
Because of their uranium and thorium content, some zircons undergo metamictization. Connected to internal radiation damage, these processes partially disrupt the crystal structure and partly explain the highly variable properties of zircon. As zircon becomes more and more modified by internal radiation damage, the density decreases, the crystal structure is compromised, and the color changes.
Zircon occurs in many colors, including reddish brown, yellow, green, blue, gray and colorless.[1] The color of zircons can sometimes be changed by heat treatment. Common brown zircons can be transformed into colorless and blue zircons by heating to 800 to 1000 °C.[12] In geological settings, the development of pink, red, and purple zircon occurs after hundreds of millions of years, if the crystal has sufficient trace elements to produce color centers. Color in this red or pink series is annealed in geological conditions above temperatures of around 400 °C.[13]
Applications

Sand-sized grains of zircon
Zircon is mainly consumed as an opacifier, and has been known to be used in the decorative ceramics industry.[14] It is also the principal precursor not only to metallic zirconium, although this application is small, but also to all compounds of zirconium including zirconium dioxide (ZrO2), one of the most refractory materials known.
Other applications include use in refractories and foundry casting and a growing array of specialty applications as zirconia and zirconium chemicals, including in nuclear fuel rods, catalytic fuel converters and in water and air purification systems.[15]
Zircon is one of the key minerals used by geologists for geochronology.
Zircon is a part of the ZTR index to classify highly-weathered sediments.
Occurrence

World production trend of zirconium mineral concentrates
Zircon is a common accessory to trace mineral constituent of most granite and felsic igneous rocks. Due to its hardness, durability and chemical inertness, zircon persists in sedimentary deposits and is a common constituent of most sands. Zircon is rare within mafic rocks and very rare within ultramafic rocks aside from a group of ultrapotassic intrusive rocks such as kimberlites, carbonatites, and lamprophyre, where zircon can occasionally be found as a trace mineral owing to the unusual magma genesis of these rocks.
Zircon forms economic concentrations within heavy mineral sands ore deposits, within certain pegmatites, and within some rare alkaline volcanic rocks, for example the Toongi Trachyte, Dubbo, New South Wales Australia[16] in association with the zirconium-hafnium minerals eudialyte and armstrongite.
Australia leads the world in zircon mining, producing 37% of the world total and accounting for 40% of world EDR (economic demonstrated resources) for the mineral.[17] South Africa is Africa’s main producer, with 30% of world production, second after Australia.[18]
Radiometric dating
Zircon has played an important role during the evolution of radiometric dating. Zircons contain trace amounts of uranium and thorium (from 10 ppm up to 1 wt%) and can be dated using several modern analytical techniques. Because zircons can survive geologic processes like erosion, transport, even high-grade metamorphism, they contain a rich and varied record of geological processes. Currently, zircons are typically dated by uranium-lead (U-Pb), fission-track, cathodoluminescence, and U+Th/He techniques. For instance, imaging the cathodoluminescence emission from fast electrons can be used as a prescreening tool for high-resolution secondary-ion-mass spectrometry (SIMS) to image the zonation pattern and identify regions of interest for isotope analysis. This is done using an integrated cathodoluminescence and scanning electron microscope.[19]Zircons in sedimentary rock can identify the sediment source.
Zircons from Jack Hills in the Narryer Gneiss Terrane, Yilgarn Craton, Western Australia, have yielded U-Pb ages up to 4.404 billion years,[20] interpreted to be the age of crystallization, making them the oldest minerals so far dated on Earth. In addition, the oxygen isotopic compositions of some of these zircons have been interpreted to indicate that more than 4.4 billion years ago there was already water on the surface of the Earth.[20][21] This interpretation is supported by additional trace element data,[22][23] but is also the subject of debate.[24][25] In 2015, "remains of biotic life" were found in 4.1 billion-year-old rocks in the Jack Hills of Western Australia.[26][27] According to one of the researchers, "If life arose relatively quickly on Earth ... then it could be common in the universe."[26]
Gallery
Crystal structure of zircon
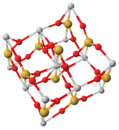
A unit cell

SEM image of zircon

Unusual olive-green zircon

Cluster of three compound crystals of zircon
Similar minerals
Hafnon (HfSiO4), xenotime (YPO4), béhierite, schiavinatoite ((Ta,Nb)BO4), thorite (ThSiO4), and coffinite (USiO4) all share the same crystal structure (VIIIX IVY O4) as zircon.
See also
Baddeleyite, ZrO2
- Cathodoluminescence microscope
- Cool Early Earth
- Earliest known life forms
- Hadean zircon
- Heavy mineral sands ore deposits
- History of Earth
- Ilmenite
References
^ abcd Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C. (eds.). "Zircon". Handbook of Mineralogy (PDF). II (Silica, Silicates). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209716..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Zircon. Mindat
^ Zircon. Webmineral
^ Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed.,
ISBN 0-471-80580-7
^ http://www.minsocam.org/MSA/AmMin/TOC/Abstracts/2013_Abstracts/Jan13_Abstracts/Erickson_p53_13.pdf
^ "Zircon definition and meaning - Collins English Dictionary". www.collinsdictionary.com. Retrieved April 29, 2018.
^ "zircon". The American Heritage Dictionary of the English Language (5th ed.). Boston: Houghton Mifflin Harcourt. 2014.
^ "Definition of ZIRCON". www.merriam-webster.com. Retrieved April 29, 2018.
^ Stwertka, Albert (1996). A Guide to the Elements. Oxford University Press. pp. 117–119. ISBN 0-19-508083-1.
^ Harper, Douglas. "zircon". Online Etymology Dictionary.
^ "Hyacinth (gem)". Encyclopædia Britannica. Encyclopædia Britannica Inc. Retrieved 7 October 2016.
^ "Zircon gemstone information". www.gemdat.org. Retrieved April 29, 2018.
^ "Integration of zircon color and zircon fission-track zonation patterns in orogenic belts: application to the Southern Alps, New Zealand". Tectonophysics. 349 (1–4): 203–219. May 6, 2002. Bibcode:2002Tectp.349..203G. doi:10.1016/S0040-1951(02)00054-9. Retrieved April 29, 2018 – via www.sciencedirect.com.
^ Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_543
^ "Products". Mineral Commodities Ltd. Retrieved 2016-08-08.
^ Staff (June 2007). "Dubbo Zirconia Project Fact Sheet June 2014" (PDF). Alkane Resources Limited. Retrieved 2007-09-10.
^ "The Mineral Sands Industry Factbook" (PDF).
^ "Heavy Minerals Mining in Africa - Titanium And Zirconium". Retrieved 2016-08-08.
^ BV, DELMIC. "Zircons - Application Note | DELMIC". request.delmic.com. Retrieved 2017-02-10.
^ ab Wilde S.A., Valley J.W., Peck W.H. and Graham C.M. (2001). "Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago" (PDF). Nature. 409 (6817): 175–8. doi:10.1038/35051550. PMID 11196637.CS1 maint: Multiple names: authors list (link)
^ Mojzsis, S.J., Harrison, T.M., Pidgeon, R.T.; Harrison; Pidgeon (2001). "Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4300 Myr ago". Nature. 409 (6817): 178–181. doi:10.1038/35051557. PMID 11196638.CS1 maint: Multiple names: authors list (link)
^ Ushikubo, T., Kita, N.T., Cavosie, A.J., Wilde, S.A. Rudnick, R.L. and Valley, J.W. (2008). "Lithium in Jack Hills zircons: Evidence for extensive weathering of Earth's earliest crust". Earth and Planetary Science Letters. 272 (3–4): 666–676. Bibcode:2008E&PSL.272..666U. doi:10.1016/j.epsl.2008.05.032.CS1 maint: Multiple names: authors list (link)
^ "Ancient mineral shows early Earth climate tough on continents". Physorg.com. June 13, 2008.
^ Nemchin, A.A., Pidgeon, R.T., Whitehouse, M.J.; Pidgeon; Whitehouse (2006). "Re-evaluation of the origin and evolution of >4.2 Ga zircons from the Jack Hills metasedimentary rocks". Earth and Planetary Science Letters. 244: 218–233. Bibcode:2006E&PSL.244..218N. doi:10.1016/j.epsl.2006.01.054.CS1 maint: Multiple names: authors list (link)
^ Cavosie, A.J., Valley, J.W., Wilde, S.A., E.I.M.F.; Valley; Wilde; e.i.m.f. (2005). "Magmatic δ18O in 4400–3900 Ma detrital zircons: a record of the alteration and recycling of crust in the Early Archean". Earth and Planetary Science Letters. 235 (3–4): 663–681. Bibcode:2005E&PSL.235..663C. doi:10.1016/j.epsl.2005.04.028.CS1 maint: Multiple names: authors list (link)
^ ab Borenstein, Seth (19 October 2015). "Hints of life on what was thought to be desolate early Earth". Excite. Yonkers, NY: Mindspark Interactive Network. Associated Press. Archived from the original on 23 October 2015. Retrieved 8 October 2018.
^ Bell, Elizabeth A.; Boehnike, Patrick; Harrison, T. Mark; et al. (19 October 2015). "Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon" (PDF). Proc. Natl. Acad. Sci. U.S.A. Washington, D.C.: National Academy of Sciences. 112: 14518–21. Bibcode:2015PNAS..11214518B. doi:10.1073/pnas.1517557112. ISSN 1091-6490. PMC 4664351. PMID 26483481. Retrieved 2015-10-20. Early edition, published online before print.
Further reading
.mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
- John M. Hanchar & Paul W. O. Hoskin (eds.) (2003). "Zircon". Reviews in Mineralogy and Geochemistry, 53.
ISBN 0-939950-65-0 (Mineralogical Society of America monograph).
D. J. Cherniak & E. B. Watson (2000). "Pb diffusion in zircon". Chemical Geology. 172: 5–24. Bibcode:2001ChGeo.172....5C. doi:10.1016/S0009-2541(00)00233-3.
A. N. Halliday (2001). "In the beginning…". Nature. 409 (6817): 144–145. doi:10.1038/35051685. PMID 11196624.
Hermann Köhler (1970). "Die Änderung der Zirkonmorphologie mit dem Differentiationsgrad eines Granits". Neues Jahrbuch Mineralogische Monatshefte. 9: 405–420.
K. Mezger & E. J. Krogstad (1997). "Interpretation of discordant U-Pb zircon ages: An evaluation". Journal of Metamorphic Geology. 15: 127–140. Bibcode:1997JMetG..15..127M. doi:10.1111/j.1525-1314.1997.00008.x.
J. P. Pupin (1980). "Zircon and Granite petrology". Contributions to Mineralogy and Petrology. 73 (3): 207–220. Bibcode:1980CoMP...73..207P. doi:10.1007/BF00381441.
Gunnar Ries (2001). "Zirkon als akzessorisches Mineral". Aufschluss. 52: 381–383.
G. Vavra (1990). "On the kinematics of zircon growth and its petrogenetic significance: a cathodoluminescence study". Contributions to Mineralogy and Petrology. 106: 90–99. Bibcode:1990CoMP..106...90V. doi:10.1007/BF00306410.
John W. Valley, William H. Peck, Elizabeth M. King, Simon A. Wilde; Peck; King; Wilde (2002). "A Cool Early Earth". Geology. 30 (4): 351–354. Bibcode:2002Geo....30..351V. doi:10.1130/0091-7613(2002)030<0351:ACEE>2.0.CO;2. Retrieved 2005-04-11.CS1 maint: Multiple names: authors list (link)
G. Vavra (1994). "Systematics of internal zircon morphology in major Variscan granitoid types". Contributions to Mineralogy and Petrology. 117 (4): 331–344. Bibcode:1994CoMP..117..331V. doi:10.1007/BF00307269.
External links
| Wikimedia Commons has media related to Zircon. |
- Geochemistry of old zircons
- Mineral galleries
GIA Gem Encyclopedia - Zircon Online articles and information on zircon history, lore, and research- Zircon Industry Association




