Jean-Pierre Changeux
Jean-Pierre Changeux | |
|---|---|
 | |
| Born | (1936-04-06) 6 April 1936 Domont, France |
| Nationality | France |
| Alma mater | École Normale Supérieure Pasteur Institute |
| Known for | MWC model, isolation of nAChR |
| Spouse(s) | Annie Dupont (m. 1962) |
| Children | 1 son |
| Awards | Wolf Prize in Medicine (1982) Louis-Jeantet Prize for Medicine (1993)[1] Sir Hans Krebs Medal (1994) Balzan Prize (2001) Albert Einstein World Award of Science (2018) |
| Scientific career | |
| Fields | Neuroscience |
| Institutions | Collège de France Institut Pasteur |
| Doctoral advisor | Jacques Monod, Francois Jacob |
Jean-Pierre Changeux (French: [ʃɑ̃ʒø]; born 6 April 1936) is a French neuroscientist known for his research in several fields of biology, from the structure and function of proteins (with a focus on the allosteric proteins), to the early development of the nervous system up to cognitive functions. Although being famous in biological sciences for the MWC model, the identification and purification of the nicotinic acetylcholine receptor and the theory of epigenesis by synapse selection are also notable scientific achievements. Changeux is known by the non-scientific public for his ideas regarding the connection between mind and physical brain. As put forth in his book, Conversations on Mind, Matter and Mathematics, Changeux strongly supports the view that the nervous system functions in a projective rather than reactive style and that interaction with the environment, rather than being instructive, results in the selection amongst a diversity of preexisting internal representations.
Contents
1 Biography
2 Scientific achievements
2.1 Allostery
2.2 Nicotinic receptor structure
2.3 Stabilization of synapses by neuronal activity
2.4 Nicotinic receptor function
2.5 Modeling cognition
3 Professional and non-scientific activities
4 Public recognition
4.1 Main scientific prizes and awards
4.2 Academic memberships and honorary degrees
4.3 Non-scientific honors
4.4 Scientific publications of historical significance
5 Books by Jean-Pierre Changeux
6 References
7 External links
Biography
Changeux was born in Domont, France to Marcel Changeux and Jean Benoit.[2] He entered the École Normale Supérieure in 1955, where he obtained a Bachelor's degree (Licence) in 1957 and a Master's degree (Diplome d'Études Supérieure) in 1958. He also received his agrégation in natural science the same year. He began his scientific career during his ENS years during summer internships in Banyuls-sur-Mer where he identified a new genus of parasitic
Copepod. He pursued PhD studies at the Pasteur Institute under the direction of Jacques Monod and Francois Jacob, and gained his doctorate in 1964. Changeux then left France for postdoctoral studies first at the University of California Berkeley (1965–1966) then at Columbia University College of Physicians and Surgeons, New-York (1967). He returned to France as attaché to the chair of Molecular Biology held by Jacques Monod. In 1972, he became director of the Unit of Molecular Neurobiology at the Pasteur Institute, where he received a professorship in 1975. In 1975, Changeux was elected professor at the Collège de France, chair of Cell Communications, position that he held until 2006. Changeux is author of more than 600 scientific articles and several books, technical or for general audience.
Scientific achievements
All his scientific career, Changeux has been faithful to a handful of scientific questions, at molecular, cellular and brain levels. If one needs to seek a unifying theme to all of them, it is the conviction that selection is the basis of life processes, rather than instruction. While started as separate lines of investigations, all the research threads were tied in the recent decades within the study of allosteric mechanisms as a basis of for the involvement of nicotinic receptors in cognitive functions.
Allostery

Diagram representing an allosteric transition of a protein between R and T states, stabilised by an Agonist, and Inhibitor and a Substrate. Adapted from Changeux and Edelstein (2004) Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition.
During his PhD studies in the laboratory of Jacques Monod and Francois Jacob, Changeux studied the allosteric regulations of enzymes, that is the modulation of their activity by compounds different from their substrates.[3][4][5] This work led to the development of the model of concerted transitions for allosteric proteins.[6][7] The main ideas behind this theory are: 1) proteins can exist under various conformations in thermal equilibrium in the absence of regulators. The allosteric regulators merely shift the equilibrium between the conformations, stabilizing the ones for which they display the highest affinity, and 2) all the subunits of a symmetrical multimeric protein exist in the same conformation, the transition taking place in a concerted fashion. The resulting model explains the observed cooperativity without a progressive change of biophysical parameters. This conceptual framework is still the principal model used to explain the function of cooperative proteins such as hemoglobin.
In his PhD thesis, Changeux suggested that the recognition and transmission of signals by membrane, and in particular by synapses, could use the same mechanisms as the allosteric regulation of enzymes. More than forty years of research would follow, mainly focussed on nicotinic acetylcholine receptors (see below). In 1967, Changeux extended the MWC model to bi-dimensional lattice of receptors[8] (an idea that would also be developed three decades afterward by Dennis Bray[9]). He then applied this idea to the post-synaptic membrane of electric organs (analog to striated muscle).[10][11] His team demonstrated the existence of several interconvertible states for the nicotinic receptor, resting, open and desensitized, displaying different affinities for the ligands, such as the endogenous agonist acetylcholine.[12][13][14] The transitions between the states followed different kinetics, and those kinetics plus the differential affinities sufficed to explain the shape of the post-synaptic potential. A full mechanistic model of the nicotinic receptor from striated muscle (or electric organ) was to be provided much later, when Changeux collaborated with Stuart Edelstein, another specialist of allostery, who worked decades on hemoglobin.[15] In addition to the allosteric modulation of the channel gating by the agonists, many other regulations of the ligand-gated ion channels activity have since been discovered. The modulators bind to a variety of allosteric sites, whether on the agonist binding sites, other binding sites at the subunit interfaces, on the cytoplasmic part of the protein or in the transmembrane domain.[16]
The concept of an allosteric pharmacology[17] for ion channels was developed over the years. In addition to the well known GABAA receptor positive allosteric modulators (such as benzodiazepines and barbiturate drugs), one can find antiparasitic drug such as ivermectin[18] and glutamate receptor modulators used against Alzheimer's disease such as aniracetam.
Nicotinic receptor structure
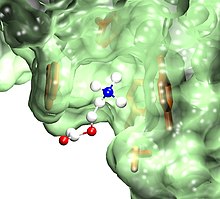
Neurotransmitter acetylcholine bound to the nicotinic acetylcholine receptor. Adapted from[19]
In 1970, Changeux isolated the nicotinic acetylcholine receptor of the eel electric organ, the first ever isolated membrane pharmacological receptor,[20] that he was able to identify thanks to the properties of a snake toxin, which was purified by Taiwanese researchers CY Lee and CC Chang.[21] The isolation of the receptor was also later reported by Ricardo Miledi.[22] The improvements of purification methods developed in the group[23] allowed the proposition that the receptor was a pentameric protein,[24] a finding quickly confirmed by the team of Arthur Karlin.[25] The group of Changeux was among the firsts to elucidate the primary structure of the subunits of the receptor,[26][27] in parallel with the group of Shosaku Numa[28] and Stephen Heinemann.[29]
Throughout the 1980s and 1990s, molecular biology technics were used to decipher the tertiary and quaternary structures of the receptor. The location of the ionic pore was identified, made up of the second transmembrane segment,[30] as shown also later by the groups of Shosaku Numa[31] and Ferdinand Hucho.[32] The molecular basis of ionic selectivity were also identified in the transmembrane domain.[33][34][35] The structure of the binding site for the acetylcholine and nicotine was located at the interface between adjacent subunits.[36][37][38]
The quest of Changeux for the structure of the nicotinic receptor culminated with the publication of the structure, at atomic resolution, of a bacterial homolog in the open[39] and resting[40] conformations supporting the concept of a symmetrical concerted opening for channel gating,[41] in agreement with molecular dynamics simulations.[42][43]
Stabilization of synapses by neuronal activity
In 1973, together with Philippe Courrège and Antoine Danchin, Changeux proposed a model describing how, during development of the nervous system, the activity of a network could cause the stabilization or regression of the synapses involved[44] and illustrated it with the neuromuscular junction. This model is effectively the precursor of the "neural Darwinism" theory further promoted by Gerald Edelman. Changeux later extended and illustrated further this idea.[45] During the 1970s, he tried to document this phenomenon, either by studying mutant animals[46][47] or by experimental denervation.[48][49]
Nicotinic receptor function

Photo by Erling Mandelmann
While until the 1990s, Changeux's group studied the structure of the nicotinic receptor present in electric organs of electric eel and torpedo, the investigations of the physiological role of those receptors were mostly focussed on two model systems: the nicotinic receptors of the neuromuscular junction, the synapse linking the motorneuron to the skeletal muscle, and the nicotinic receptors of the brain, notably in relation with nicotine addiction.
From the mid-1980s, the group studied the compartimentalisation of the muscle cell upon development, as a model of synaptogenesis and in relation with the theoretical work on epigenesis. In particular, the group focussed on the accumulation of nicotinic receptors in the post-synaptic region upon development, concommitent to a switch of receptor identity. They were able to decrypt the different signalling pathways involved in the response to synaptic activity, showing that the accumulation resulted from an inhibition of gene transcription outside the synaptic region due to electrical activity triggering an uptake of calcium and activation of PKC,[50][51][52][53] and a stimulation of gene transcription at the synapse by the calcitonin gene-related peptide (CGRP) activating PKA[54][55][56] and the ARIA (heregulin) activating tyrosine kinase cascades.[57][58]
The 1990s saw the progressive shift of interest of Changeux from the neuromuscular junction to the nicotinic receptors expressed in the brain. Among the notable achievements of the group is the discovery that neuronal nicotinic receptors are highly permeable to calcium[59] - which explains the positive effect of nicotinic receptors on the release of many neurotransmitters in the brain.[60]
The group also discovered that the nicotinic receptor is regulated by a variety of "allosteric modulators" such as: 1. calcium ions[61] (This was also discovered independently by the group of John Dani[62]), which binding sites were later identified[19][63] and localized in the extracellular domain, at the interface between subunits (Le Novère et al. 2002); 2. ivermecin[18] which behaves as a potent positive allosteric modulator binding to a site present in the transmembrane domain (where general anesthetics also bind[64]); 3. phosphorylation of the cytoplasmic domain[65] which regulate desensitization.
By the mid-1990s, Changeux concentrated most of his interest on the function of nicotinic receptors in the basal ganglia and in particular the mesencephalic dopaminergic system. Using mice deleted for nicotinic receptor genes, the group characterised the types of receptor subunits present in the dopaminergic cells[66][67][68] and identified the receptors mainly responsible of the dependence to nicotine, formed by the subunits α4, α6 and β2.[69][70]
Modeling cognition
From the mid-1990s, Changeux developed an activity of computational modeling in order to investigate the neuronal bases of cognitive functions. This research was mainly performed in collaboration with Stanislas Dehaene, now leading the INSERM-CEA Cognitive Neuroimaging Unit. They notably modeled the acquisition of song recognition in birds[71] and the development of numerical abilities.[72] More recently, Dehaene and Changeux developed a neuronal model for access to consciousness based on a brain-wide recruitment of networks of neurons with long-range axons, referred to as the global neuronal workspace.[73][74] The model might have clinical applications for instance for understanding the mechanism of coma, the action of general anesthetics[75] or drug addiction[76]
Professional and non-scientific activities
The publication of his book Neuronal Man: The Biology of The Mind in 1985 brought Changeux celebrity status among the wider public. Since then, he authored or co-authored several other books inspired by his teaching at the College de France: notably, Conversations on Mind Matter and Mathematics with the mathematician Alain Connes (1998), What Makes Us Think with the philosopher Paul Ricoeur (2002) and the Physiology of truth (2002) that are acknowledged as having initiated an instructive dialogue between the two often-hostile disciplines of neuroscience and philosophy. He has also been concerned by the relationships between aesthetic experience and the brain in Raison & Plaisir (1994), The true the good the beautiful: a neurobiological approach (2012) and recently Les neurones enchantés. (2014) where he debates the issue of artistic creation with the music composers Pierre Boulez and Philippe Manoury. Changeux received the Lewis Thomas Prize for Writing about Science, Rockefeller University, New-York, 2005.
Changeux has also been the curator of three major exhibitions on Art and Science: De Nicolo dell'Abate à Nicolas Poussin: aux sources du Classicisme 1550-1650 Musée Bossuet Meaux in 1988, L'Âme au Corps, Arts et Sciences, 1793-1993 (with Gérard Régnier) Galeries nationales du Grand Palais Paris in 1993-1994 and La lumière au siècle des Lumières et aujourd'hui. Art et science : de la biologie de la vision à une nouvelle conception du monde Galeries Poirel Nancy in 2005. Changeux has also chaired the inter-ministry commission for the conservation of the French artistic heritage since 1989, and has been member of the scientific council of the International Agency of museums since 2007.
Last, throughout his career, Changeux has been concerned by the ethical consequences for the city and for the society in general of the recent progress in the Neuroscience. Changeux has headed the National Advisory Committee on Bioethics in France from 1992 to 1998. He organised a scientific conference on the topic, that led to a book he edited, fondements naturel de l'ethique. He is presently the co-chairman of the Ethics and Society division of the European Human Brain Program (since 2013).
He is also on the Board of Scientific Governors of The Scripps Research Institute, an independent non for profit institute focusing on biomedical research.
Public recognition
Main scientific prizes and awards
- 1978: Gairdner Foundation International Award
- 1982: Wolf Foundation Prize in Medicine
- 1982: prize Richard-Lounsbery of the US Academy of Sciences and the French Academy of Sciences
- 1991: Carl-Gustav-Bernhard medal of the Swedish Academy of Science
- 1992: CNRS Gold medal.
- 1993: Louis-Jeantet Prize for Medicine
- 1994: Goodman and Gilman Award in drug receptor pharmacology
- 1998: Prize Emanuel Merck in Chemistry, Darmstadt
- 2001: Balzan Prize for Cognitive Neurosciences
- 2002: American Philosophical Society's Karl Spencer Lashley Award in neuroscience
- 2005: Lewis Thomas Prize for Writing about Science
- 2005: Golden Eurydice Award
- 2007: NAS Award in the Neurosciences from the National Academy of Sciences[77]
- 2012 Japanese Society for the Promotion of Science (JSPS) award for eminent scientists
- 2016: International research award from the Olav Thon Foundation (Oslo)[78]
- 2018: Albert Einstein World Award of Science conferred by the World Cultural Council[79]
Academic memberships and honorary degrees
Deutsche Akademie der Naturforscher Leopoldina zu Halle (Pharmacology), 1974 ; Académie de Médecine de Turin, 1976 ; National Academy of Sciences, Washington (US) (foreign associate), 1983 ; Royal Academy of Sciences, Stockholm, (Sweden) (foreign member), 1985 ; Académie des Sciences, Paris, 1988 ; Académie Royale de Médecine de Belgique (Bruxelles) (foreign honorary member), 1988 ; Academia Europaea (founding member), 1988 ; American Academy of Arts and Sciences, Boston, (US) (foreign member), 1994 ; Romanian Academy of Medical Sciences, Bucarest (foreign member), 1996 ; Institute of Medicine of the National Academies, Washington, (US) (foreign associate), 2000 ; Istituto Veneto di Scienze, Lettere Ed Arti, Venezia (Italy), 2001 ; Hungarian Academy of Sciences, Budapest (foreign member associate), 2004 ; European Academy of Sciences, Bruxelles (member), 2004 ; International Academy of Humanism; Académie Royale des Sciences, des Lettres & des Beaux-Arts de Belgique (foreign member), 2010; Accademia Nazionale dei Lincei, Rome, (Italy) (foreign member), 2010.
Doctor honoris causa : Universities of Torino, Italy, 1989 ; Dundee, Scotland, 1992 ; Geneva, Switzerland, 1994 ; Stockholm, Sweden, 1994 ; Liège, Belgium, 1996 ; Ecole Polytechnique Fédérale of Lausanne, Switzerland, 1996 ; University of Southern California, Los Angeles, US, 1997 ; Bath, UK, 1997 ; Montréal University, Canada, 2000 ; The Hebrew University of Jerusalem, Israel, 2004 ; Ohio State University, Columbus, US, 2007; University of Buenos
Aires, Argentina, 2010.
Honorary member of Neurosciences Research Program, MIT and Rockefeller University (US), since 1984; Honorary member of the Japanese Biochemical Society, Sendai, Japan, 1985 ; Honorary member of the American Neurology Association, 1988 ; Honorary member of University College London, 1990 ; Membre d'honneur à titre étranger de la Société Belge de Neurologie, Bruxelles, 1991 ; Member of European Molecular Biology
Organization.
Non-scientific honors
Grand Croix dans l'Ordre de la Légion d'Honneur, 2010; Grand-Croix dans l'Ordre National du Mérite 1995 ; Commandeur dans l'Ordre des Arts et des Lettres, 1994.
Scientific publications of historical significance
Monod, J.; Wyman, J.; Changeux, J. P. (1965). "On the Nature of Allosteric Transitions: A Plausible Model". Journal of Molecular Biology. 12: 88–118. doi:10.1016/S0022-2836(65)80285-6. PMID 14343300..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em} (in which Jacques Monod, Jeffries Wyman and Jean-Pierre Changeux presented the concerted model of allosteric transitions, that explained the cooperativity exhibited by many allosteric proteins, such as hemoglobin)
- Changeux J.-P., Kasai M., Huchet M., Meunier J.-C. (1970). Extraction à partir du tissu électrique de gymnote d'une protéine présentant plusieurs propriétés caractéristiques du récepteur physiologique de l'acétylcholine. C. R. Acad. Sci. 270D: 2864-2867. (the first purification of a neurotransmitter receptor. Since the article is in French, most people quote the description of the toxin that allowed the receptor to be identified: Changeux, J.; Kasai, M.; Lee, C. (1970). "Use of a snake venom toxin to characterize the cholinergic receptor protein". Proceedings of the National Academy of Sciences of the United States of America. 67 (3): 1241–1247. doi:10.1073/pnas.67.3.1241. PMC 283343. PMID 5274453.
Changeux, J.; Courrège, P.; Danchin, A. (1973). "A theory of the epigenesis of neuronal networks by selective stabilization of synapses". Proceedings of the National Academy of Sciences of the United States of America. 70 (10): 2974–2978. doi:10.1073/pnas.70.10.2974. PMC 427150. PMID 4517949. (In which the authors develop a formal model of synapse selection, precursor of the "neural darwinism". This is the original work, although most people quote the subsequent review [better suited to a non-specialist audience and presenting the biological context]: Changeux JP, Danchin A (1976) Nature, 264 (1976) 705—712.)
Books by Jean-Pierre Changeux
- Changeux, Jean-Pierre. (2008) Du vrai, du beau, du bien : Une nouvelle approche neuronale
- Changeux, Jean-Pierre; Stuart Edelstein. (2004) Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition
- Changeux, Jean-Pierre. (2002) L'homme de verite (2004 The physiology of truth)
- Changeux, Jean-Pierre; Paul Ricœur. (1998) Ce qui nous fait penser (2002 What Makes Us Think. A Neuroscientist and a Philosopher Argue About Ethics, Human Nature, and the Brain[80][81])
- Changeux, Jean-Pierre. (1994) Raison et plaisir
- Changeux, Jean-Pierre; Alain Connes. (1989) Matière à pensée (1995 Conversations on Mind, Matter and Mathematics)
- Changeux, Jean-Pierre. (1983) L'homme neuronal (1985 Neuronal Man: The Biology of Mind)
References
^ Louis-Jeantet Prize
^ The International Who's Who 2004. Psychology Press. 2003. p. 299. ISBN 9781857432176.
^ Changeux J.-P. (1961). The feedback control mechanism of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harbor. Symp. Quant. Biol. 26: 313-318.
^ Changeux J.-P. (1963). Allosteric Interactions on biosynthetic L-theonine deaminase from E. coli K12. Cold Spring Harb Symp Quant Biol, 28: 497-504
^ Monod J., Changeux J.-P., and Jacob. F. (1963). Allosteric proteins and cellular control systems. J. Mol. Biol. 6: 306-329
^ Monod J., Wyman J., and Changeux J.-P. (1965). On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12: 88-118.
^ Rubin M.M., Changeux J.-P. (1966). On the nature of allosteric transitions ; implications of non-exclusive ligand binding. J. Mol. Biol. 21: 265-274.
^ Changeux J.-P., Thiéry J.-P., Tung Y., and Kittel C. (1967). On the cooperativity of biological membranes. Proc. Natl. Acad. Sci. USA 57, 335-341.
^ Bray D, Levin MD, Morton-Firth CJ (1998) Receptor clustering as a cellular mechanism to control sensitivity. Nature, 393: 85-88.
^ Changeux J.-P., Podleski T.R. (1968). On the excitability and cooperativity of electroplax membrane. Proc. Natl. Acad. Sci. USA 59:944-950
^ Cartaud J., Benedetti E.L., Cohen J.B., Meunier J.C., Changeux J.-P. (1973) Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata. FEBS Lett. 33: 109-113.
^ Weber M., David-Pfeuty M.T., Changeux J.-P. (1975). Regulation of binding properties of the nicotinic receptor protein by cholinergic ligands in membrane fragments from Torpedo marmorata. Proc. Natl. Acad. Sci. USA 72: 3443-3447.
^ Sugiyama H., Changeux J.-P. (1975). Interconversion between different states of affinity for acetylcholine of the cholinergic receptor protein from Torpedo marmorata. Eur. J. Biochem. 55: 505-515.
^ Heidmann T., Changeux J.-P. (1979). Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from T. marmorata. Eur. J. Biochem. 94: 255-279.
^ Edelstein S., Schaad O., Henry E., Bertrand D. Changeux J.-P. (1996). A kinetic mechanism for nicotinic acetylcholine receptors based on multiple allosteric transitions. Biol. Cybern. 75: 361-379
^ Changeux J.-P. (2012). The concept of allosteric modulation: an overview. Drug Discov. Today 10: e223-e228
^ Christopoulos, A; Changeux, J. P.; Catterall, W. A.; Fabbro, D; Burris, T. P.; Cidlowski, J. A.; Olsen, R. W.; Peters, J. A.; Neubig, R. R.; Pin, J. P.; Sexton, P. M.; Kenakin, T. P.; Ehlert, F. J.; Spedding, M; Langmead, C. J. (2014). "International Union of Basic and Clinical Pharmacology. XC. Multisite pharmacology: Recommendations for the nomenclature of receptor allosterism and allosteric ligands". Pharmacological Reviews. 66 (4): 918–47. doi:10.1124/pr.114.008862. PMID 25026896.
^ ab Krause, R. M.; Buisson, B; Bertrand, S; Corringer, P. J.; Galzi, J. L.; Changeux, J. P.; Bertrand, D (1998). "Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor". Molecular Pharmacology. 53 (2): 283–94. PMID 9463487.
^ ab Le Novère N., Grutter T., Changeux J.-P. (2002). Models of the extracellular domain of the nicotinic receptors and of agonist and Ca++ binding sites. Proc. Natl. Acad. Sci. USA, 99: 3210-3215.
^ Changeux J.-P., Kasai M., Huchet M., Meunier J.-C. (1970). Extraction à partir du tissu électrique de gymnote d'une protéine présentant plusieurs propriétés caractéristiques du récepteur physiologique de l'acétylcholine. C. R. Acad. Sci. 270D: 2864-2867.
^ Changeux J.-P., Kasai M., and Lee C.Y. (1970). The use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Natl. Acad. Sci. USA 67: 1241-1247.
^ Miledi R., Molinoff P., Potter L.T. (1971). Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature 229:554-557.
^ Olsen R., Meunier J.C., Changeux J.-P. (1972). Progress in purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett. 28., 96-100.
^ Hucho F., Changeux J.-P. (1973). Molecular weight and quaternary structure of the cholinergic receptor protein extracted by detergents from Electrophorus electricus electric tissue. FEBS Lett. 38: 11-15
^ Weill C.L., McNamee M.G., Karlin A. (1974) Affinity-labeling of purified acetylcholine receptor from Torpedo Californica. Biochem Biophys Res Comm 61: 997-1003.
^ Devillers-Thiéry A., Changeux J.-P., Paroutaud P., and Strosberg A.D. (1979). The amino-terminal sequence of the 40.000 molecular weight subunit of the acetylcholine receptor protein from Torpedo marmorata. FEBS Lett. 104: 99-105.
^ Devillers-Thiéry A., Giraudat J., Bentaboulet M., Changeux J.-P. (1983). Complete mRNA coding sequence of the acetylcholine binding alpha subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc. Natl. Acad. Sci. USA 80: 2067-2071.
^ Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. (1982) Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature 299:793-797.
^ Ballivet M., Patrick J., Lee J., Heinemann S. (1982) Molecular cloning of cDNA coding for the gamma subunit of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 79:4466-4470.
^ Giraudat J., Dennis M., Heidmann T., Chang J.Y., Changeux J.-P. (1986). Structure of the high affinity site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]-chlorpromazine. Proc. Natl. Acad. Sci. USA 83: 2719-2723.
^ Imoto K., Methfessel C., Sakmann B., Mishina M., Mori Y., Konno T., Fukuda K., Kurasaki M., Bujo H., Fujita Y., Shosaku N. (1986). Location of a delta-subunit region determining ion transport through the acetylcholine receptor channel. Nature. 1986 Dec 18-31;324(6098):670-4.
^ Hucho F., Oberthür W., Lottspeich F. (1986) The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett.205: 137-142.
^ Galzi J.-L., Devillers-Thiery A., Hussy N., Bertrand S., Changeux J.-P., Bertrand D. (1992). Mutations in the ion channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature 359: 500-505.
^ Bertrand D., Galzi J.-L., Devillers-Thiéry A., Bertrand S., Changeux J.-P. (1993). Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha7 nicotinic receptor. Proc. Natl. Acad. Sci. USA 90: 6971-6975.
^ Corringer P.-J., Bertrand S., Galzi J.-L., Devillers-Thiéry A., Changeux J.-P., Bertrand D. (1999). Mutational Analysis of the Charge Selectivity Filter of the a7 Nicotinic Acetylcholine Receptor. Neuron 22: 831-843.
^ Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J.Y., Lazure C., Chrétien M., Changeux J.-P. (1988). Amino acids of the Torpedo marmorata acetylcholine receptor subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry 27: 2346-2357.
^ Galzi J.-L., Revah F., Black D., Goeldner M., Hirth C., Changeux J.-P. (1990). Identification of a novel amino acid a-Tyr 93 within the active site of the acetylcholine receptor by photoaffinity labeling: additional evidence for a three-loop model of the acetylcholine binding site. J. Biol. Chem. 265: 10430-10437.
^ Galzi J.-L., Bertrand D., Devillers-Thiéry A., Revah F., Bertrand S., Changeux J.-P. (1991). Functional significance of aromatic amino acids from three peptide loops of the alpha 7 neuronal nicotinic receptor site investigated by site-directed mutagenesis. FEBS Lett. 294: 198-202.
^ Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J.-P., Delarue M., Corringer P.-J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open
conformation. Nature 457(7225):111-114
^ Sauguet, L; Shahsavar, A; Poitevin, F; Huon, C; Menny, A; Nemecz, A.; Haouz, A; Changeux, J. P.; Corringer, P. J.; Delarue, M (2014). "Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation". Proceedings of the National Academy of Sciences. 111 (3): 966–71. doi:10.1073/pnas.1314997111. PMC 3903189. PMID 24367074.
^ Hilf R.J., Dutzler R. (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457(7225):115-118
^ Taly A., Delarue M., Grutter T., Nilges M., Le Novère N., Corringer P.-J., Changeux J.-P. (2005) Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys. J. 88:3954-3965
^ Calimet N., Simoes M., Changeux J.-P., Karplus M., Taly A., Cecchini M. (2013) From the Cover: A gating mechanism of pentameric ligand-gated ion channels. Proc Natl Acad Sci U S A. 110:E3987-3996
^ Changeux J.-P., Courrège P., Danchin A. (1973). A theory of the epigenesis of neural networks by selective stabilization of synapses. Proc. Natl. Acad. Sci. USA 70: 2974-2978.
^ Changeux J.-P., Danchin, A. (1976). Selective stabilization of developing synapses as a mechanism for the specification of neuronal networks. Nature 264: 705-712.
^ Sotelo C., Changeux J.-P. (1974). Transsynaptic degeneration 'en cascade' in the cerebellar cortex of staggerer mutant mice. Brain Res. 67: 519-526.
^ Mariani J., Crepel F., Mikoshiba K., Changeux J.-P. (1977). Anatomical, physiological and biochemical studies of the cerebellum from reeler mutant mouse. Phyl. Trans. Royal Soc. B 281: 1-28
^
Benoit P, Changeux J.P. (1975) Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res.99:354-8
^ Henderson CE, Huchet M, Changeux JP. Denervation increases a neurite-promoting activity in extracts of skeletal muscle. Nature. 1983 Apr 14;302(5909):609-11.
^ Betz H., Changeux J.-P. (1979). Regulation of muscle acetylcholine receptor synthesis in vitro by cyclic nucleotide derivatives. Nature 278: 749-752.
^ Klarsfeld A., Changeux J.-P. (1985). Activity regulates the level of acetylcholine receptor alpha-subunit mRNA in cultured chick myotubes. Proc. Natl. Acad. Sci. USA 82: 4558-4562.
^ Klarsfeld A., Laufer R., Fontaine B., Devillers-Thiéry A., Dubreuil C., Changeux J.-P. (1989). Regulation of muscle AChR alpha-subunit gene expression by electrical activity : involvement of protein kinase C and Ca++. Neuron 2: 1229-1236.
^ Piette J., Bessereau J.-L., Huchet M., Changeux J.-P. (1990). Two adjacent MyoD1-binding sites regulate the expression of the acetylcholine receptor delta-subunit gene. Nature 345: 353-355.
^ Fontaine B., Klarsfeld A., Hokfelt T., Changeux J.-P. (1986). Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci. Lett. 71: 59-65.
^ Fontaine B., Klarsfeld A., Changeux J.-P. (1987). Calcitonin-gene related peptide and muscle activity regulate acetylcholine receptor alpha-subunit mRNA levels by distinct intracellular pathways. J. Cell Biol. 105: 1337-1342.
^ Laufer R., and Changeux J.-P. (1987). Calcitonin gene-related peptide elevates cyclic AMP levels in chick skeletal muscle : possible neurotrophic role for a coexisting neuronal messenger. EMBO J. 6: 901-906.
^ Altiok N., Bessereau J.-L., Changeux J.-P. (1995). ErB3 and ErbB2/neu mediate the effect of heregulin on acetylcholine receptor gene expression in muscle : differential expression at the endplate. EMBO J. 14: 4258-4266.
^ Schaeffer L., Duclert N., Huchet-Dymanus M., Changeux J.-P. (1998). Implication of a multisubunit Ets related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J., 17: 3078-3090.
^ Mulle C., Choquet D., Korn H., Changeux J.-P. (1992). Calcium influx through nicotinic receptor in rat central neurons : Its relevance to cellular regulation. Neuron 8: 135-143.
^ Léna C, Changeux, JP (1997). Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci 17: 576-585.
^ Mulle C., Léna C., Changeux J.-P. (1992). Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron 8: 937-945.
^ Vernino S, Amador M, Leutje CW, Patrick J, and Dani JA (1992) Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron 8: 127-134
^ Galzi J.-L., Bertrand S., Corringer P.-J., Changeux J.-P., Bertrand D. (1996). Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J. 15: 5824-5832.
^ Nury H., Van Renterghem C., Weng Y., Tran A., Baaden M., Dufresne V., Changeux J.-P., Sonner J.M., Delarue M., Corringer P.-J. (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469(7330):428-431
^ Teichberg V.I., Sobel A., Changeux J.-P. (1977) In vitro phosphorylation of the acetylcholine receptor. Nature 267(5611):540-542
^ Le Novère N., Zoli M., Changeux J.-P. (1996). Neuronal nicotinic receptor a6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci 8: 2428-2439
^ Klink R., de Kerchove d'Exaerde A., Zoli M., Changeux J.-P. (2001). Molecular and Physiological Diversity of Nicotinic Acetylcholine Receptors in the Midbrain Dopaminergic Nuclei. J. Neurosci. 21: 1452-1463.
^ Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci., 2003 Aug 27;23(21):7820-9.
^ Picciotto M.R., Zoli M., Rimondini R., Léna C., Marubio L., Merlo Pich E., Fuxe K., Changeux J.-P. (1998). Acetylcholine receptors containing the b2-subunit are involved in the reinforcing properties of nicotine. Nature 391: 173-177 (1998).
^ Maskos U., Molles B.E, Pons S., Besson M., Guiard B.P., Guilloux J.P., Evrard A., Cazala P., Cormier A., Mameli-Engvall M., Dufour N., Cloz-Tayarani I., Bemelmans A.-P., Mallet J., Gardier A.M., David V., Faure P., Granon S. and Changeux J.-P. (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436: 103-107
^ Dehaene S., Changeux J.-P., Nadal J.P. (1987). Neural networks that learn temporal sequences by selection. Proc. Natl. Acad. Sci. USA 84: 2727-2731.
^ Dehaene S., Changeux J.-P. (1993). Development of elementary numerical abilities : a neuronal model. J. Cognitive Neurosci 5: 390-407.
^ Dehaene S., Kerszberg M., Changeux J.-P. (1998). A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci USA 95: 14529-14534.
^ Dehaene S., Sergent C., Changeux J.-P. (2003) A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc. Natl. Acad. Sci. USA, 100: 8520-8525.
^ Changeux J.-P. (2012) Conscious processing: implications for general anesthesia. Curr. Opin. Anesthesiol. 25:397–404.
^ Changeux J.-P., Lou H.C. (2011) Emergent pharmacology of conscious experience: new perspectives in substance addiction. FASEB J. 25(7):2098-2108.
^ "NAS Award in the Neurosciences". National Academy of Sciences. Archived from the original on 29 December 2010. Retrieved 16 February 2011.
^ "International research award from the Olav Thon Foundation 2016". Retrieved 28 April 2016.
^ "Le prix Albert-Einstein World Award of Science 2018 est remis à Jean-Pierre Changeux" (in French). Collège de France. 4 June 2018. Retrieved 3 July 2018.
^ Review of What Makes Us Think by Howard Gardner Archived 16 February 2005 at the Wayback Machine
^ Review of What Makes Us Think by Elliott White Archived 22 March 2006 at the Wayback Machine
External links
- Jean-Pierre Changeux's Google Scholar page
- Jean-Pierre Changeux's laboratory in 2005
Jean-Pierre Changeux International Balzan Prize Foundation